PHARMACOVIGILANCE
11,Dec 2019 Mir Shahroz
For establishment of Pharmacovigilane system, Safety database, Case processing, Literature search etc,
mail: info@medwisdom.in; Phone: +91-9264127040
Pharmacovigilance system (INDIA).
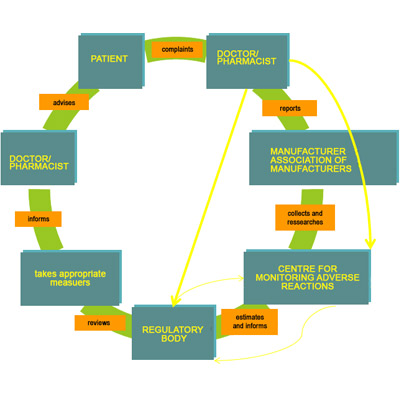
As we seen in the past that there are several Tragedies/Adverse effects (e.g., Thalidomide, Alosetron, Anagestone acetate, Diethylstilbestrol, Gemtuzumab ozogamicin etc.1) has been happened related with the drugs. The drug has been marketed after evaluation of it safety efficacy and quality by the drug regulator based on the benefit risk ratio. But In spite of these regulatory activities, we see the serious adverse effects of drugs in post marketing surveillance.
As per the World health organization (WHO), Pharmacovigilance is defined as follows:
2Pharmacovigilance (PV) is defined as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem.
2The aims of Pharmacovigilance are to enhance patient care and patient safety in relation to the use of medicines; and to support public health programmes by providing reliable, balanced information for the effective assessment of the risk-benefit profile of medicines.
ADRs (Adverse drug reactions) are one of the leading causes of morbidity and mortality:
- Up to 35% of hospitalized patients experience an ADR
- Approximately 5% to 10% of all hospital admissions are due to ADR Over 2 Million serious ADRs yearly
- 1,00,000 deaths yearly due to ADRs
- ADRs are 4th leading cause of death ahead of pulmonary disease, diabetes mellitus and AIDS
- Drug related deaths account for the fourth largest category of mortality in the United States. [4]
- In USA, estimated total cost of medication errors and adverse drug reactions (ADRs) exceed 136 billion dollars annually. [5]
- Greater than total costs of cardiovascular or diabetic care
- Mean length of stay, cost and mortality for ADR patients are DOUBLE that for control patients
Pharmacovigilance system in INDIA
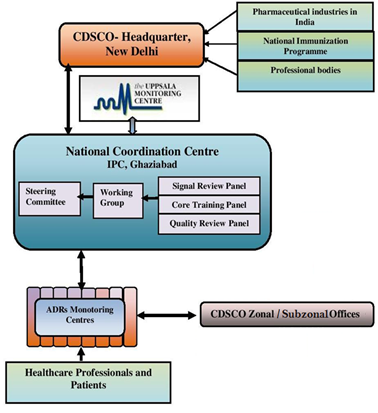
The Pharmacovigilance activities to determine the drug safety has been monitored by the Indian Pharmacopoeia commission {Pharmacovigilance Programme of India (PvPI)3}. The Pharmacovigilance Programme of India (PvPI) was started by the Government of India on 14th July 2010 with the All India Institute of Medical Sciences (AIIMS), New Delhi as the National Coordination Centre for monitoring Adverse Drug Reactions (ADRs) in the country for safe-guarding Public Health. In the year 2010, 22 ADR monitoring centres including AIIMS, New Delhi was set up under this Programme. To safeguard implementation of this programme in a more effective way, the National Coordination Centre was shifted from the All India Institute of Medical Sciences (AIIMS), New Delhi to the Indian Pharmacopoeia Commission, Ghaziabad, Uttar Pradesh on 15th April 2011.
Data Collection:
Data has been collected in form of ICSR, PSUR, RMP by the Pharmacovigilance Programme of India (PvPI).
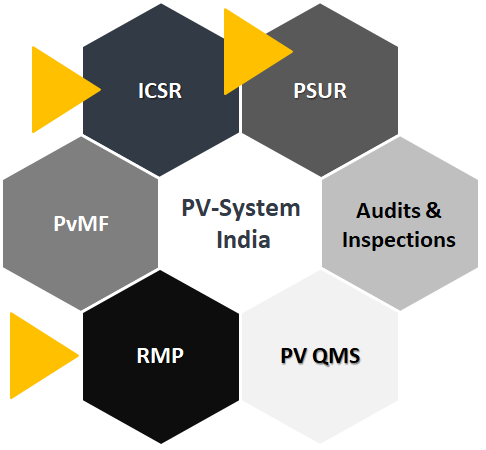
ICSR (Individual Case Study Report) management:
The adverse events due to pharmaceutical products (if any) to their preferred ADRs monitoring centre by using following (Refer: http://www.pvpi.in/adr.html):
-
Suspected ADRs reporting form (for HCPs)
-
ADR reporting form for Kala Azar
-
Medicines side effect reporting form for patients in Hindi & other Vernacular Languages
-
Mobile app (Download Mobile Application from Google Play Store)
-
Helpline Number Managed by NCC PvPI to provide assistance in ADRs reporting for the HCPs and general public: 1800 180 3024 (All Working Days 9:00 AM to 5:30PM)
References:
-
Onakpoya et al. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature; BMC Medicine (2016) 14:10.
-
https://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/
-
US FDA. ADRs: Prevalence and incidence. [Last accessed on 2014 Jan 20]. Available from: http://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/druginteractionslabeling/ucm110632.htm .
-
Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med. 1995;155:1949–56.







