Some Latest & Important Post
Recent Posts
13,Dec 2019 Mir Shahroz
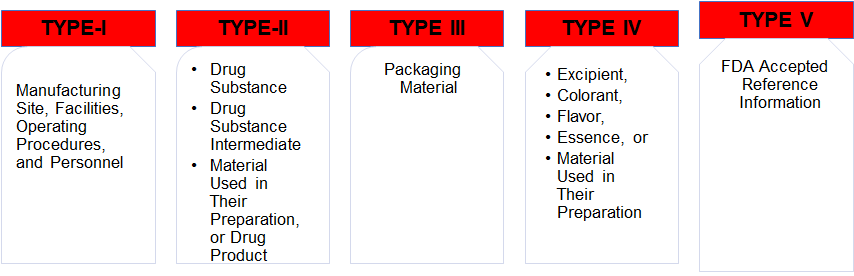
DRUG MASTER FILE (US-FDA)
DRUG MASTER FILE (US-FDA SUBMISSION)
18,Nov 2019 Mir Shahroz
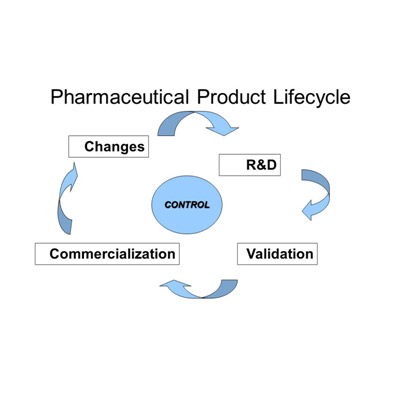
POST APPROVAL
Once approval has been obtained, there is usually a need for variations on the original marketing authorisation
19,Nov 2019 Mir Shahroz
Medical Devices
Classification of medical device, preparation of technical master file, Clinical and non-clical expert report of medical device and in vitro diagnostic.

18,Nov 2019 Mir Shahroz
DUE DILIGENCE
Are you contemplating a licensing deal, a strategic partnership or a merger & acquisition
15,Dec 2019 Mir Shahroz
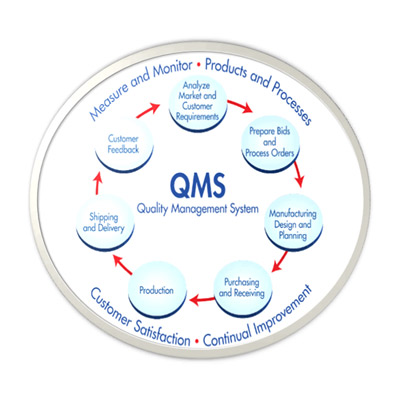
QMS/cGMP Audit Preparation and Establishment
QMS/cGMP Audit Preparation and Establishment
for Pharmaceutical/Neutraceuticals/Herbal/Medical device/Excipients/Drug Substance company
GAP analysis, System establishment, Facilitation of Regulatory Agency Audit, Deficiency response (Agency) etc,
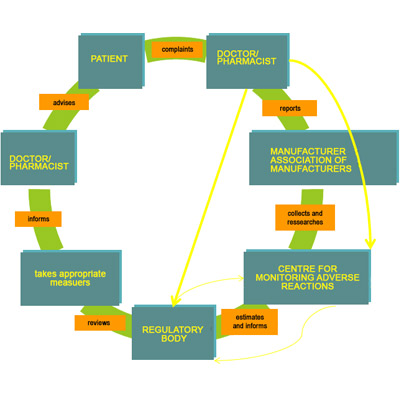
11,Dec 2019 Mir Shahroz
PHARMACOVIGILANCE
Pharmacovigilance system (INDIA)
Looking an Adequate Solution for your Company?
Contact us today for free consultation or more information
Get In TouchCopyright © 2022 | Medwisdom.in | All Rights Reserved






